FDA Official In Charge Of Evaluating New Drugs Hospitalized For ‘Mental Disorder’
Posted: Thu Jun 02, 2022 5:49 pm
https://www.dailywire.com/news/fda-offi ... k-goodness
Lashes out that his "job carries a lot of responsibilities -- I'm an office director at FDA making major decisions every day that impact the public health."


Jeffrey Siegel, MD,
Director, Office of Drug Evaluation Sciences, Office of New Drugs, CDER
FDA, United States
Jeffrey Siegel is the director of the Office of Drug Evaluation Sciences (ODES) in the Office of New Drugs (OND), CDER, FDA. ODES oversees Clinical Outcome Assessments, Biomarker Qualification, Research and Bioinformatics in OND. Dr Siegel has over 20 years of experience in research, regulatory, and clinical drug development. Jeff received his B.A. from Columbia University and M.D. from Yale University. Following his training in internal medicine and basic science research, he served at FDA from 1996-2010 as a medical officer and then Medical Team Leader. In 2010, he left FDA for industry and worked at Genentech/Roche and subsequently at Gilead Sciences before rejoining FDA in February, 2021.
Source: https://www.diaglobal.org/en/conference ... s/speakers
Lashes out that his "job carries a lot of responsibilities -- I'm an office director at FDA making major decisions every day that impact the public health."

A top federal official in charge of evaluating the safety of drugs was hospitalized against his will this month for an unspecified “mental disorder,” prompting concern over his fitness for the role he said includes making “major decisions that impact on public health,” The Daily Wire has learned.
Dr. Jeffrey Siegel, director of the Food and Drug Administration’s Office of Drug Evaluation Sciences, was transported from his home to a hospital for “mental disorder” at 3 a.m. on May 9, according to Montgomery County, Maryland, police dispatch logs reviewed by The Daily Wire. Police declined to release a report on the incident despite a Freedom of Information Act request, but Siegel wrote about being taken from his home in a rambling note left on a neighborhood listserv.
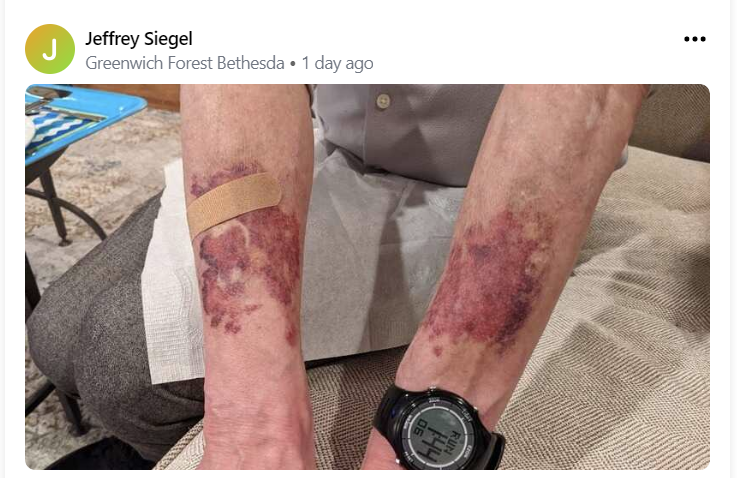
“15 minutes later I saw bright red and blue lights (I may have the colors wrong) outside the house and then EMTs in our house who started to ask me to please come with them,” Siegel wrote in the note, more than a week after his hospitalization. “Thinking I was being asked to go voluntarily I politely declined. They insisted. Finally, they turned me around in the steps of my front hallway on Moorland Lane, handcuffed me, forced me unto a gurney, tied me down, took me in the ambulance to the hospital.”
One neighbor who received the missive via Nextdoor expressed concern about Siegel’s ability to do his job, given that Siegel also wrote that he makes ‘major decisions that impact on public health.'
“I am frankly left a little bit worried for the soundness of operations at our FDA, if indeed that is true,” the neighbor wrote.
A biography of Siegel states that he leads the office that “oversees Clinical Outcome Assessments, Biomarker Qualification, Research and Bioinformatics” in the Office of New Drugs. An FDA org chart says that the deputy director position is vacant, and that he is also leading the “Division of Biomecial [sic] Informaci [sic], Research & Biomaker [sic] Dev.”
Siegel posted the note with pictures of bruised wrists, apparently sustained during a struggle with paramedics who were restraining him, complained about “brain fog,” and lashed out out a particular doctor for not giving him drugs he was seeking.
Siegel did not respond to requests for comment.
In his note, Siegel boasted how his “job carries a lot of responsibilities.”
“Finally, I realized this was not voluntary but had been ordered by someone against my will… so I stopped resisting. When in the hospital I was given a clean bill of heath [sic] including CT and MRI and they sent me home on Tuesday with no diagnosis,” he wrote.
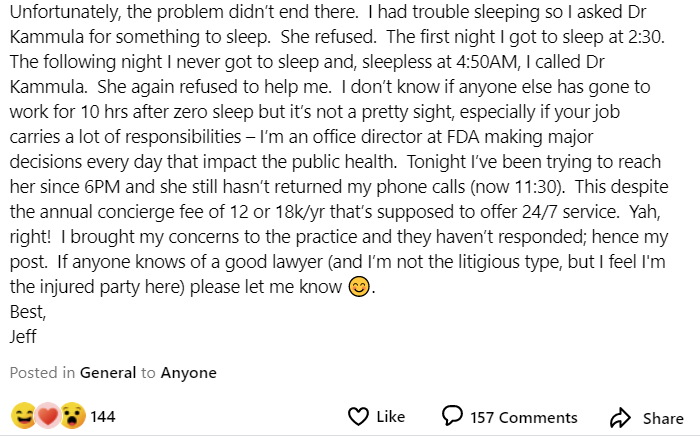
“Unfortunately, the problem didn’t end there. I had trouble sleeping so I asked [the doctor] for something to sleep. She refused,” he said. He claimed he paid her practice between $12,000 and $18,000 a year and asked “if anyone knows of a good lawyer (and I’m not the litigious type, but I feel I’m the injured party here).”
Neighbors responded by questioning his account, with saying that someone had filed an “emergency petition” against him, and another speculating that it was his wife, not the doctor.
“This glass of juice has leaks in it, I would suggest a psychologist, rather than a lawyer. Good luck getting the mental health help you need,” another said.
Siegel, for his part, commented on the thread that he had “No COVID. Thank goodness.”
A Science.org article titled “FDA’s revolving door: Companies often hire agency staffers who managed their successful drug reviews,” published in 2018, reported that Siegel back in 2010 oversaw the approval of an arthritis drug from Genentech, and months later left the agency to join the company and represented it “before his former FDA colleagues when the company sought approval” to promote the drug for other conditions. He said the timing of his decision was coincidental.
Jeffrey Siegel, MD,
Director, Office of Drug Evaluation Sciences, Office of New Drugs, CDER
FDA, United States
Jeffrey Siegel is the director of the Office of Drug Evaluation Sciences (ODES) in the Office of New Drugs (OND), CDER, FDA. ODES oversees Clinical Outcome Assessments, Biomarker Qualification, Research and Bioinformatics in OND. Dr Siegel has over 20 years of experience in research, regulatory, and clinical drug development. Jeff received his B.A. from Columbia University and M.D. from Yale University. Following his training in internal medicine and basic science research, he served at FDA from 1996-2010 as a medical officer and then Medical Team Leader. In 2010, he left FDA for industry and worked at Genentech/Roche and subsequently at Gilead Sciences before rejoining FDA in February, 2021.
Source: https://www.diaglobal.org/en/conference ... s/speakers